(Numbers next to atoms are their formal charges)
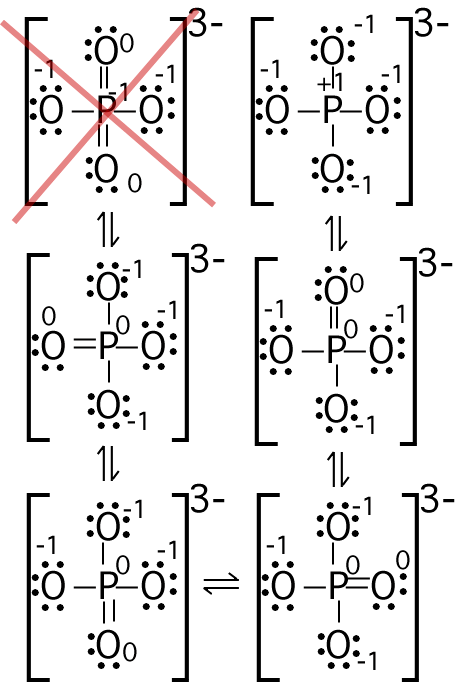
Starting at the upper right corner and
moving clockwise we can make 4 resonance structures that expand the P
octet to 10. In all of these the P has a formal charge of 0 and
one oxygen is also 0. The last resonance structure expands the P
octet to 12, but is not a very good structure because the less
electronegative P has a more negative formal charge then some of the
O. The best resonance structures are the four at the bottom.