(numbers next to atoms are their formal charges)
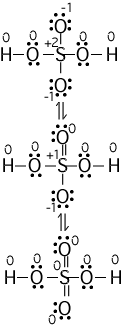
The bottom resonance structure is the
best because all the formal charges are zero. We could go on
expanding the S octet, but this would start to move the formal charges
away from zero; thus we stop here.