(numbers next to atoms are their formal charges)
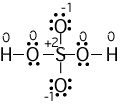
Example formal charge calculations:
S--> 6 - (1/2)8 = +2, O without H --> 6 - 6 - (1/2)2 = -1, O with
H --> 6 - 4 - (1/2)4 = 0. Notice that the sum of the absolute
values of the formal charges is 4, although this species is
uncharged. This suggests that the Lewis structure is not very
good. We will see how to make it better in step 8.