Models of Br2 were made using the best ab initio level of theory, which was double zeta valance, DZV. This is the best level of theory because it is the highest basis set of calculations run though it for the molecule.
Bond lengths of Br2 were found using the ab initio levels.
| Optimized
Geometry
|
Bond
Length
(Br-Br) Å |
| 321G |
2.443
Å |
| 631G |
2.406
Å |
| DZV |
2.426
Å |
|
|
The experimental value for Br2 is 2.281 Å.1
Br2 is a linear molecule.
The HOMO orbital is the highest energy molecular orbital occupied by electrons. From the model, it is seen that the HOMO orbital of Br2 is non-bonding, and from this model a person can predict how a molecule will react. Click below for the electrical surface of bromine, and on BROMOHOMO for the HOMO orbital.
|
|
|
|
BROMOLUMO should, at this juncture, be self explanatory.
|
|
Other types of calclations run...
The dipole moments for Br2 are all zero because dipole moments are the measure of the separation of positive and negative electrical charges in a system of charges and the dibromine has an even distribution of charge because they are the same at each end.
The vibrational frequency for Br2 the DZV level was calculated as 310.0 cm-1. The Br2 vibrational frequency from the NIST website was 325.0 cm-1.
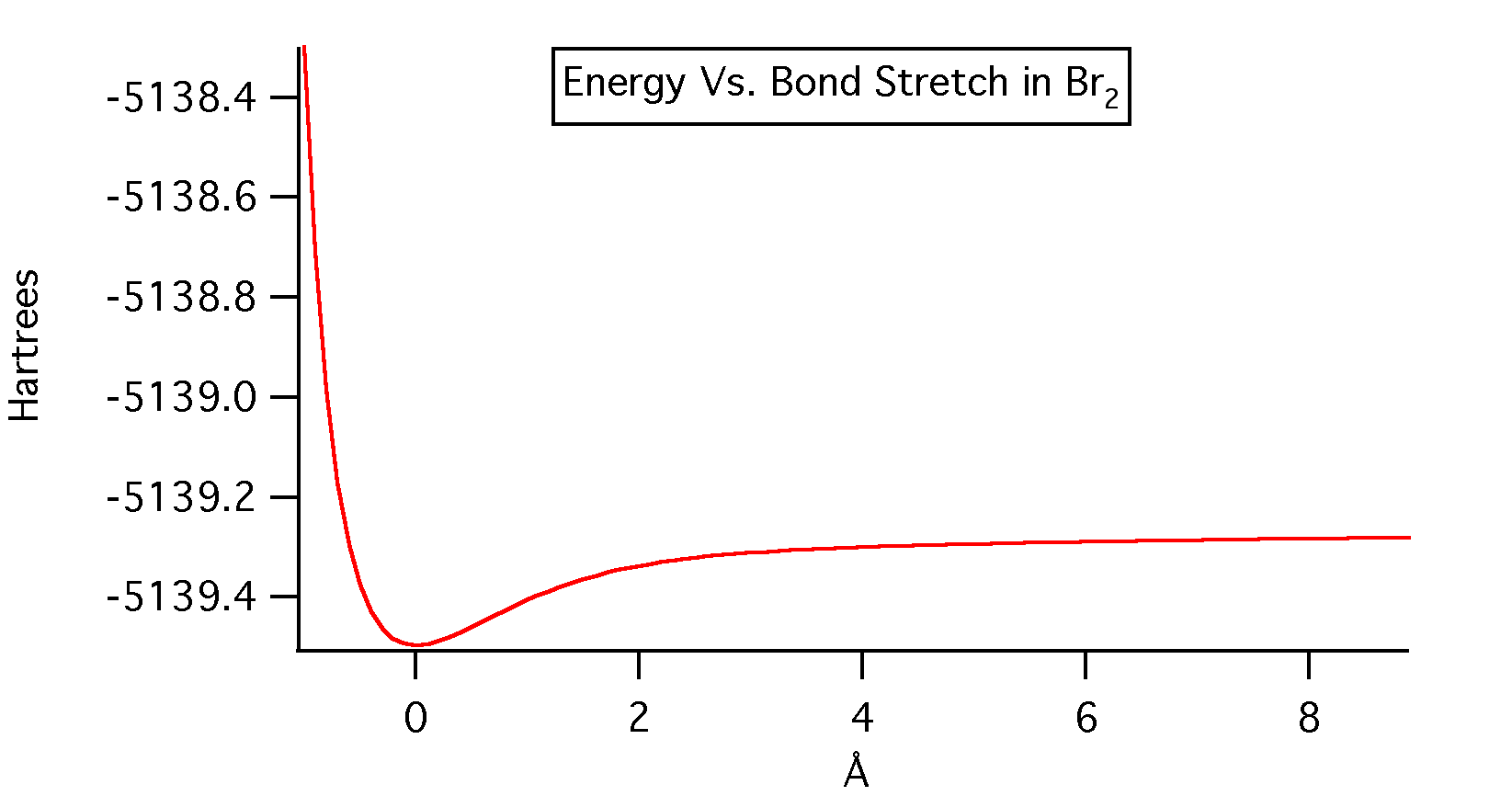
Potential of bond stretching for the DZV level of theory. This is the highest level of theory that was used to calculate the potential bond stretching of the molecule.
|
|
Reference
(1) U.S. Secretary of Commerce, Computational Chemistry Comparison and Benchmark
DataBase, on behalf of the United States of America, 2010. Website: <http://cccbdb.nist.gov/>
Page skeleton
and JavaScript generated by export to web function using Jmol 11.8.20 2010-02-28 19:28
on Mar 24, 2010.